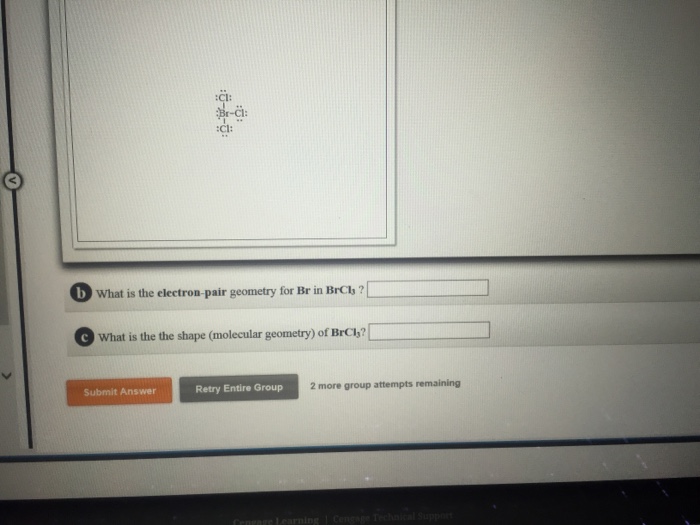
What Is the Electron Pair Geometry for Br in Brcl3
Answer Solved Subscribe To Get Solution. Carbon dioxide CO2 lon Chlorite ion CIO2 Lewis Structure.

Solved The Following Drawings Of The Electron Domain Picture Chegg Com
How many lone pairs.

. There are lone pair s around the central atom so the molecular geometry shape of H2O is B. What is the electron-pair geometry for O in H2O. Chemistry questions and answers.
What is the electron-pair geometry for br in brcl3. May 21 2021 View more View Less. Ground state configuration of boron- 1s2 2s2 2p1.
The molecular geometry of Boron Trichloride BCl3 is a trigonal planar with asymmetrical charge distribution around the central atom. What is the electron-pair geometry for Be in Bel2. The total number of valence electrons will be 28 7 from each of the three chlorine atoms and 7 from the bromine atom.
Out of the 28 valence electrons 24 will be used to complete the octets of the chlorine atoms - each chlorine atom. Electron pair geometry molecular shape polar or nonpolar valence. The electron-domain geometry of PF6 is Octahedral since the central atom Phosphorus has an electron pair geometry which is octahedral What it the electron geometry for PF3.
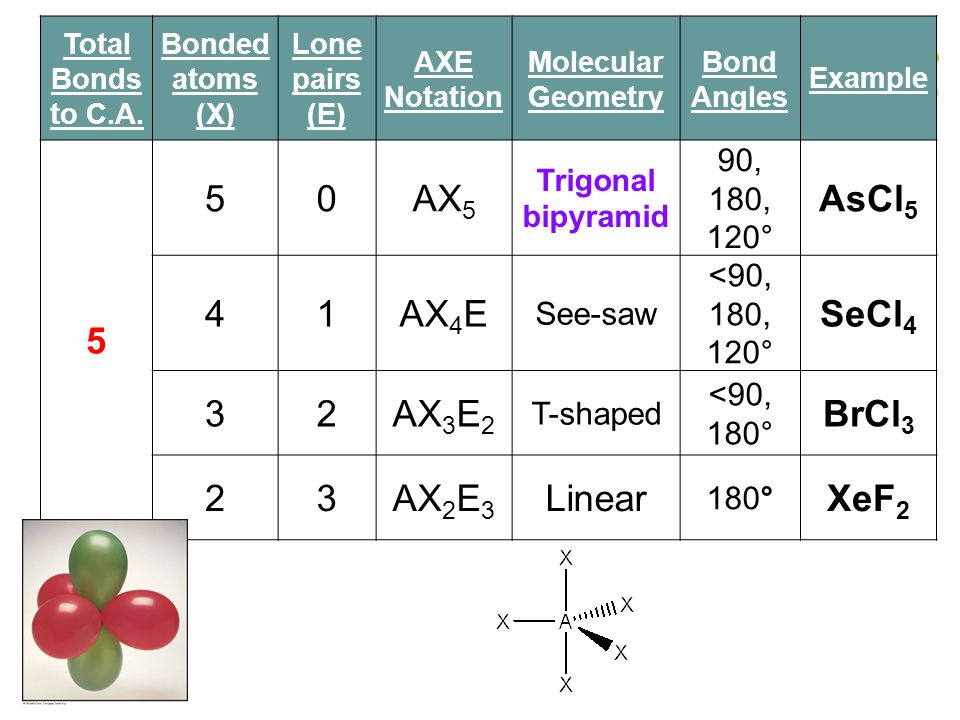
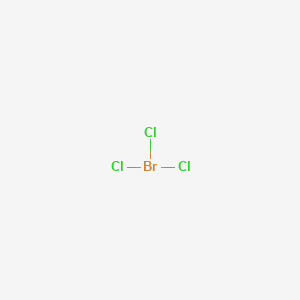
The molecular geometry of SF 5 Cl is octahedral and the charge distribution on the central atom is asymmetric. No of lone pair 2 no of bond pair 3 total no of bond pairs 23 5 HYBRIDIZATION Br sp3d geometry trigonal bipyramidal shape t- shape. In bromine trichloride or BrCl3 the bromine atom is sp3d hybridized.
There are 2 lone pair s around the central atom so the molecular geometry shape of BrCl 3 is T-shaped. The electron geometry Electronic Domain Geometry for PF3 is tetrahedral. What is the electron pair geometry.
Up to 256 cash back Molecule Lewis nyl COC12 Structure. Sp 3 d 2. Start by drawing the Lewis structure of the compound.
The electron-pair geometry of Br in BrCl3 is trigonal bipyramidal. Ion Hydronium ion H30 Lewis Structure valence electrons pairs lone pairs sigma bonds electron pair molecular shape or valence electrons pairs lone pairs sigma bonds. What is the molecular geometry.
What is the lewis structure for BrCl3. An explanation of the molecular geometry for the BrCl5 ion Bromine pentachloride including a description of the BrCl5 bond angles. The electron geometry fo.
There are lone pairs around the central atom so the molecular geometry shape of brcl3 is - 27178879. However when a molecule is polar then even when it is a trigonal planar shape it cant have a bond angle of exactly 120 degree. There are lone pair s around the central atom so the molecular geometry shape of Bel2 is A.
Answer 1 of 4.

Brcl3 Lewis Structure How To Draw The Lewis Structure For Brcl3 Bromine Trichloride Youtube

Brcl3 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Brcl3 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
What Is The Hybridization Of Brcl3 Quora
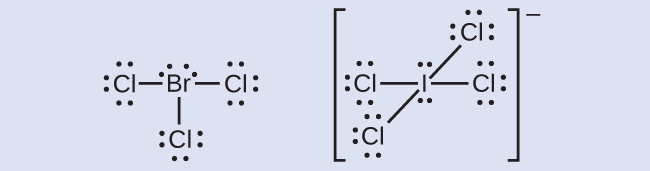
10 1 Lewis Structures And The Octet Rule Chemistry Libretexts
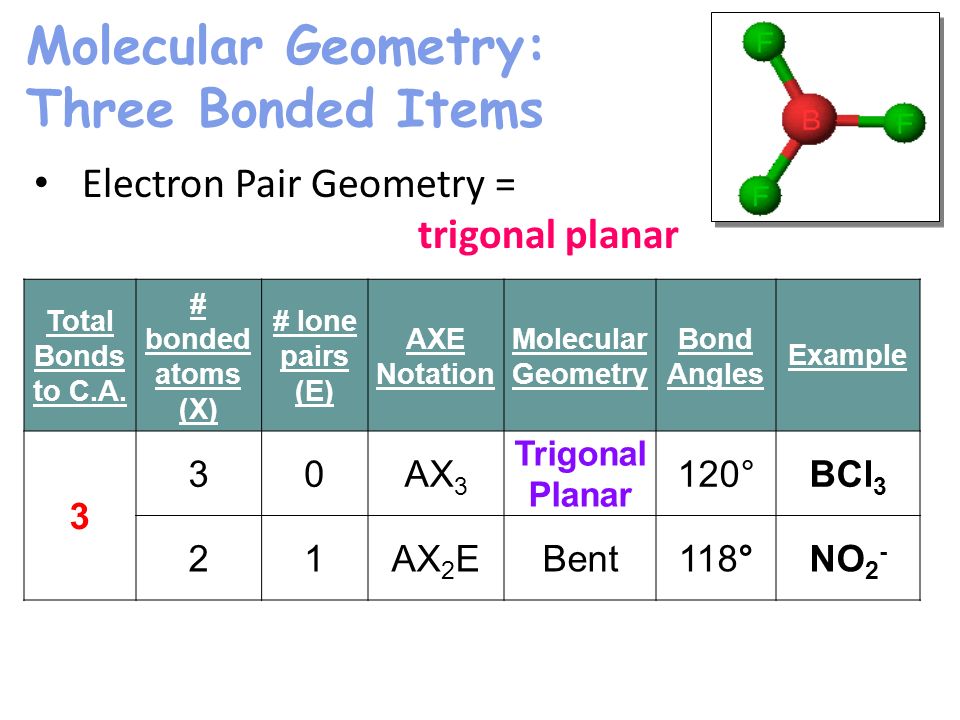
Unit 7 Bonding Molecular Geometry Ppt Video Online Download

Is Brcl3 Polar Or Nonpolar Bromine Trichloride Youtube
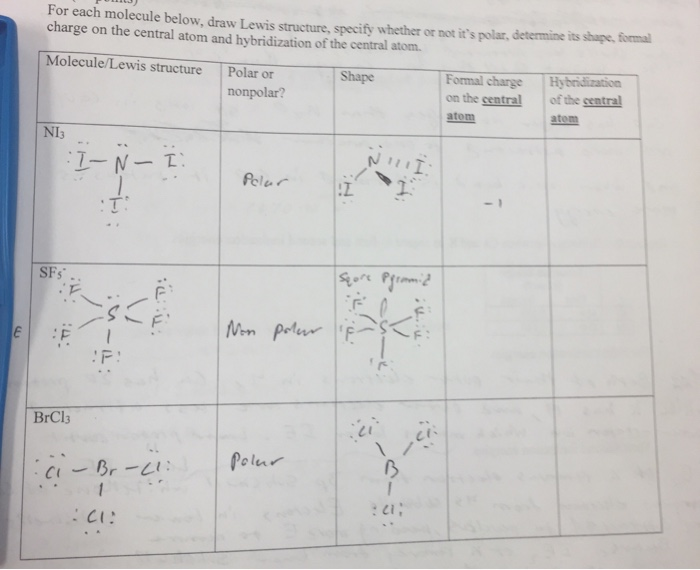
Solved For Each Molecule Below Draw Lewis Structure Chegg Com

Brcl3 Lewis Structure Bromine Trichloride Youtube

Brcl3 Lewis Structure How To Draw The Lewis Structure For Brcl3 Bromine Trichloride Youtube
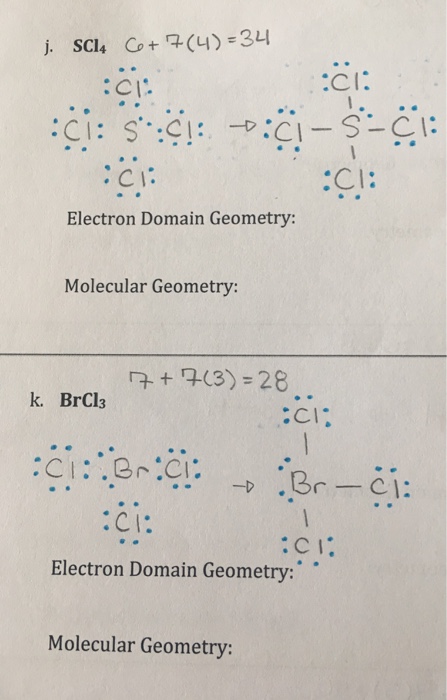
Solved Scl 4 Electron Domain Geometry Molecular Geometry Chegg Com

Brcl3 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Brcl3 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Brcl3 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
Unit 7 Bonding Molecular Geometry Ppt Video Online Download
Solved What Is The Electron Pair Geometry For Br In Chegg Com

Brcl3 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Comments
Post a Comment